Latest News
 Obiri, Lemma claim Boston Marathon
Obiri, Lemma claim Boston Marathon
 Dinner over death in Concord
Dinner over death in Concord
 Another Chipotle coming to Concord
Another Chipotle coming to Concord
 Portsmouth temple again targeted with vandalism
Portsmouth temple again targeted with vandalism

Vandals key cars outside NHGOP event at Concord High; attendee carrying gun draws heat from school board
Vandals keyed the cars of at least 10 members of the state Republican Party while the group held its biennial convention inside Concord High School on Saturday. “There’s already too much vitriol in partisan politics,” Party Chair Chris Ager said. Ager...

Former Franklin High assistant principal Bill Athanas is making a gift to his former school
Bill Athanas saw the sign outside Laconia High School and flashed back to the hallways of Franklin High.He remembered walking past the plain Franklin High sign – white letters on a black background, flanked by two small brick pillars – and greeting...
Most Read
 FAFSA fiasco hits New Hampshire college goers and universities hard
FAFSA fiasco hits New Hampshire college goers and universities hard
 Opinion: New Hampshire, it’s time to drive into the future
Opinion: New Hampshire, it’s time to drive into the future
 Hometown Hero: Quilters, sewers grateful for couple continuing ‘treasured’ business
Hometown Hero: Quilters, sewers grateful for couple continuing ‘treasured’ business
 Steeplegate Mall redevelopment, with Cosco and Whole Foods as well as 625 housing units, to be heard next week
Steeplegate Mall redevelopment, with Cosco and Whole Foods as well as 625 housing units, to be heard next week
 Red barn on Warner Road near Concord/Hopkinton line to be preserved
Red barn on Warner Road near Concord/Hopkinton line to be preserved
Editors Picks
 Concord martial arts studio builds life skills far beyond combat
Concord martial arts studio builds life skills far beyond combat
 Red barn on Warner Road near Concord/Hopkinton line to be preserved
Red barn on Warner Road near Concord/Hopkinton line to be preserved
 Hometown Hero: Quilters, sewers grateful for couple continuing ‘treasured’ business
Hometown Hero: Quilters, sewers grateful for couple continuing ‘treasured’ business
 Searchable Concord salary database: Top earners include more police, fewer women
Searchable Concord salary database: Top earners include more police, fewer women
Sports

High schools: Concord boys’ lax earns first win, plus more weekend results
Boys’ Lacrosse Concord 9, Dover 8 Key players: Concord – Carter Doherty (5 goals), Jaden Haas (4 goals), Brayden Beauregard (3 assists), Tyler Morin (assist), Ben Ryder (assist), Logan Shimer (goalie), Noah Wyatt (defense), Broc Pelchat (defense),...
 High schools: Wednesday and Thursday’s results
High schools: Wednesday and Thursday’s results
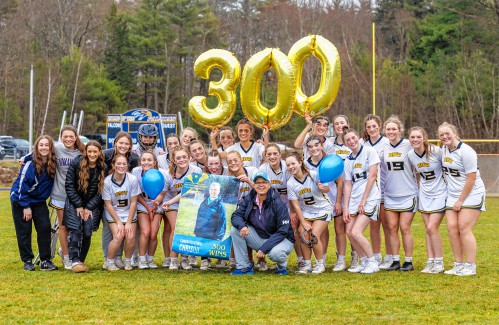 Bow girls’ lacrosse coach Chris Raabe achieves milestone win
Bow girls’ lacrosse coach Chris Raabe achieves milestone win
 High schools: Bow girls’ lax coach Raabe wins 300th game, more Wednesday results
High schools: Bow girls’ lax coach Raabe wins 300th game, more Wednesday results
 Boys’ lacrosse: Hopkinton and Bow head to overtime in season opener
Boys’ lacrosse: Hopkinton and Bow head to overtime in season opener
Opinion

Opinion: Members of NH Jewish community write letter to NH congressional delegation
Bob Sanders lives in Concord. On a rainy April 10, I with the help of one other delivered a message on behalf of 67 New Hampshire Jews to the office of Rep. Annie Kuster in Concord, calling for a ceasefire in Gaza, humanitarian aid, and cutting off...
 Opinion: Whatever a court decides, Elizabeth Gurley Flynn retains an important place in American labor history
Opinion: Whatever a court decides, Elizabeth Gurley Flynn retains an important place in American labor history
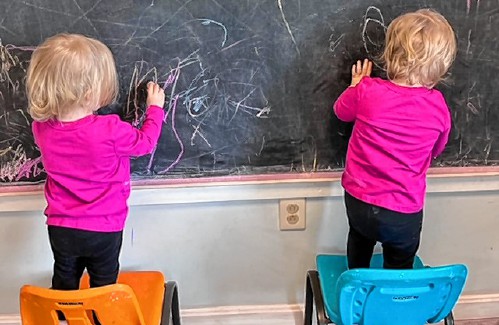 Opinion: How our twin toddlers turned our lives (and chairs) upside down
Opinion: How our twin toddlers turned our lives (and chairs) upside down
 Opinion: New Hampshire, it’s time to drive into the future
Opinion: New Hampshire, it’s time to drive into the future
 Opinion: Eid al-Fitr in Gaza: A war against humanity itself
Opinion: Eid al-Fitr in Gaza: A war against humanity itself

Politics

House passes bill removing exceptions to state voter ID law
The House narrowly approved a bill Thursday that would eliminate any exceptions to the state’s voter ID laws and require documentary proof of citizenship to vote, 189-185. The bill, House Bill 1569, would require a person registering to vote to...
 League of Women Voters suing over AI robocalls sent in NH
League of Women Voters suing over AI robocalls sent in NH
 As NH lawmakers eye statewide housing solutions, local control remains a barrier
As NH lawmakers eye statewide housing solutions, local control remains a barrier
 On the trail: Haley was first in and last out
On the trail: Haley was first in and last out
 Haley will suspend her campaign and leave GOP to Trump
Haley will suspend her campaign and leave GOP to Trump
Arts & Life

Vintage Views: The greatest factory that never was
It was almost two hundred years ago when our Concord ancestors thought and planned and then they planned and thought again. They dreamed about what could be and about the riches that might follow. A period of time commonly referred to as the Second...
 Inspired by Robert Frost, New Hampshire Poet Laureate Jennifer Militello has achieved her childhood dreams
Inspired by Robert Frost, New Hampshire Poet Laureate Jennifer Militello has achieved her childhood dreams
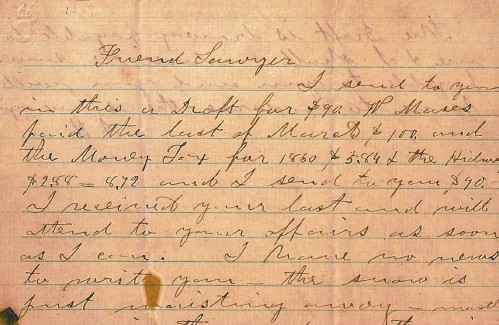 From the archives: Civil War brewing
From the archives: Civil War brewing
 From the farm: Celebrate springtime
From the farm: Celebrate springtime
 National Water Dances comes to NH
National Water Dances comes to NH
Obituaries
 Winnifred H. Bailey
Winnifred H. Bailey
Boscawen, NH - Winnifred H. Bailey, age 81, passed away peacefully on Tuesday, April 9, 2024 at Merrimack County Nursing Home. She was born in Concord, NH the daughter of the late Cecil an... remainder of obit for Winnifred H. Bailey
 Richard Fleury
Richard Fleury
Allenstown, NH - Richard Fleury, 80, of Allenstown, passed away on Easter, March 31, 2024 at The Pines of Newmarket, where he lived for the past 2 years due to care he needed for dementia/A... remainder of obit for Richard Fleury
 Robert L. Wickham
Robert L. Wickham
[IMAGE]Robert L. Wickham Concord, NH - Surrounded by those he held dear, Robert L. Wickham died April 12, 2024, at the venerable age of 96. He loved his family, his country, music, animals ... remainder of obit for Robert L. Wickham
 Jean V. Lawrie
Jean V. Lawrie
Concord, NH - Jean Lawrie of Concord, NH passed away peacefully at the age of 73 at the Merrimack County Nursing Home in Boscawen on March 22, 2024. Jean was born on October 17, 1950 in... remainder of obit for Jean V. Lawrie

 Baseball: Concord makes eight errors but shows reasons for optimism in wild extra-inning loss
Baseball: Concord makes eight errors but shows reasons for optimism in wild extra-inning loss
 High schools: Monday’s baseball, softball, lacrosse and tennis results
High schools: Monday’s baseball, softball, lacrosse and tennis results
 Two democrats with parallel views run for same State Senate seat
Two democrats with parallel views run for same State Senate seat
 Granite Geek: Forest streams are so pretty; too bad they’re such a pain to measure
Granite Geek: Forest streams are so pretty; too bad they’re such a pain to measure
 NH resident pleads guilty to selling stolen body parts
NH resident pleads guilty to selling stolen body parts


