
Students’ first glimpse of new Allenstown school draws awe
Jaws dropped and cheers erupted as Allenstown students entered their new school building for the first time Thursday morning.“Yo, this looks like a mall,” said an eighth grade boy in a Bruins shirt. “Smell that new school smell,” observed another.“We...

‘Bridging the gap’: Phenix Hall pitch to soften downtown height rules moves forward
Sue McCoo knew that adding flexibility to downtown zoning to allow for the repurposing of Phenix Hall would mean her store, Hilltop Consignment, would probably lose its longtime home on Main Street. The zoning change would open the door for the...
Most Read
 Regal Theater in Concord is closing Thursday
Regal Theater in Concord is closing Thursday
 With less than three months left, Concord Casino hasn’t found a buyer
With less than three months left, Concord Casino hasn’t found a buyer
 Phenix Hall, Christ the King food pantry, rail trail on Concord planning board’s agenda
Phenix Hall, Christ the King food pantry, rail trail on Concord planning board’s agenda
 Former Franklin High assistant principal Bill Athanas is making a gift to his former school
Former Franklin High assistant principal Bill Athanas is making a gift to his former school
 Another Chipotle coming to Concord
Another Chipotle coming to Concord
 Generally speaking, Don Bolduc, now a Pittsfield police officer, has tested himself for years
Generally speaking, Don Bolduc, now a Pittsfield police officer, has tested himself for years
Editors Picks
 Concord martial arts studio builds life skills far beyond combat
Concord martial arts studio builds life skills far beyond combat
 Red barn on Warner Road near Concord/Hopkinton line to be preserved
Red barn on Warner Road near Concord/Hopkinton line to be preserved
 Hometown Hero: Quilters, sewers grateful for couple continuing ‘treasured’ business
Hometown Hero: Quilters, sewers grateful for couple continuing ‘treasured’ business
 Searchable Concord salary database: Top earners include more police, fewer women
Searchable Concord salary database: Top earners include more police, fewer women
Sports

High schools: Tuesday’s track, baseball, softball, lacrosse and tennis results
Boys’ TrackMerrimack Valley 110.33, Bow 101.66, Pembroke 44, St. Thomas 41, Bishop Brady 35Key players: MV – Nicolas Ogelsby (1st high jump, 1st long jump), Brayden Laroche (1st 100 hurdles, 1st 300 hurdles)), Mychal Reynolds (1st 400), Abhiman Karki...
 High schools: Monday’s baseball, softball, lacrosse and tennis results
High schools: Monday’s baseball, softball, lacrosse and tennis results
 Obiri, Lemma claim Boston Marathon
Obiri, Lemma claim Boston Marathon
Opinion

Opinion: Bankers have the NH Public Deposit Investment Pool in their sights
Todd Selig is the longtime town manager in Durham. Senate Bill 553 would require public funds from localities be invested in NH banks, where the NH Bankers Association, the lobbying arm for the banks, argues that according to a study it commissioned,...
 Opinion: Proposed height zoning change for Concord’s Main Street
Opinion: Proposed height zoning change for Concord’s Main Street

Politics

Sununu says he’ll support Trump even if he’s convicted
As jury selection begins this week in the hush-money trial of former President Donald Trump, New Hampshire Gov. Chris Sununu says he doesn’t believe many voters view Trump’s criminal indictments, his actions on Jan. 6, 2021, or his election denialism...
 NH mayors want more help from state on homelessness prevention funds
NH mayors want more help from state on homelessness prevention funds
 Two democrats with parallel views run for same State Senate seat
Two democrats with parallel views run for same State Senate seat
 House passes bill removing exceptions to state voter ID law
House passes bill removing exceptions to state voter ID law
 League of Women Voters suing over AI robocalls sent in NH
League of Women Voters suing over AI robocalls sent in NH
Arts & Life

NH Furniture Masters present new member show this spring
The NH Furniture Masters are pleased to feature the work of three new members in our spring exhibition: Dan Faia, Mike Korsak, and Philip Morley. This exhibit celebrates the creativity and dedication of our newest members and brings together a diverse...
 Vintage Views: The greatest factory that never was
Vintage Views: The greatest factory that never was
 Inspired by Robert Frost, New Hampshire Poet Laureate Jennifer Militello has achieved her childhood dreams
Inspired by Robert Frost, New Hampshire Poet Laureate Jennifer Militello has achieved her childhood dreams
 From the archives: Civil War brewing
From the archives: Civil War brewing
Obituaries
 Arthur R. Stern
Arthur R. Stern
Belmont, NH - Arthur "Sonny" Stern, 82, passed on Friday, April 12, 2024, while returning from wintering in Florida. He was born on March 8, 1942, in Frederick Maryland. Sonny was the o... remainder of obit for Arthur R. Stern
 Barbara J. McCaffrey
Barbara J. McCaffrey
Barbara J. (Smith) McCaffrey Concord, NH - Barbara J. (Smith) McCaffrey, died peacefully on Monday, April 15, 2024, at the age of 97, after a period of declining health. She was born in Conc... remainder of obit for Barbara J. McCaffrey
 Larraine E. Butler
Larraine E. Butler
Loudon, NH - Larraine Butler, 77 years old, of Loudon, New Hampshire, passed away peacefully at home surrounded by her family. Larraine leaves behind her husband, James F. Butler, who she ... remainder of obit for Larraine E. Butler
 Jane A. Haskell
Jane A. Haskell
Boscawen , NH - Jane A. Haskell, 95, died peacefully on Saturday April 13, 2024, at Merrimack County Nursing Home. She was born in Dover, NH, the daughter of Amy (Towle) and Harold Brow... remainder of obit for Jane A. Haskell

 Mother of two convicted of negligent homicide in fatal Loudon crash released on parole
Mother of two convicted of negligent homicide in fatal Loudon crash released on parole
 After delay over neighbors’ concerns, Christ the King Food Pantry headed for rebuild
After delay over neighbors’ concerns, Christ the King Food Pantry headed for rebuild
 Casinos can no longer charge rent to charities
Casinos can no longer charge rent to charities
 Man granted parole for his role in the 2001 stabbing deaths of 2 Dartmouth College professors
Man granted parole for his role in the 2001 stabbing deaths of 2 Dartmouth College professors
 ‘I was scared:’ Meehan takes the stand in lawsuit alleging NH enabled child abuse at YDC
‘I was scared:’ Meehan takes the stand in lawsuit alleging NH enabled child abuse at YDC
 ‘It all feels so magical’: New England college students celebrate an LGBTQ prom at Dartmouth
‘It all feels so magical’: New England college students celebrate an LGBTQ prom at Dartmouth
 Opinion: Being and becoming: A good doctor in the age of artificial intelligence
Opinion: Being and becoming: A good doctor in the age of artificial intelligence
 Wednesday’s high schools: Fancher’s 2-run blast leads Concord baseball to victory; plus more baseball, softball, lax and tennis results
Wednesday’s high schools: Fancher’s 2-run blast leads Concord baseball to victory; plus more baseball, softball, lax and tennis results
 Softball: Maddy Wachter Ks 12, Concord holds off Winnacunnet in 2023 championship rematch
Softball: Maddy Wachter Ks 12, Concord holds off Winnacunnet in 2023 championship rematch

 Boys’ lacrosse: With a different level of energy and focus, MV feels primed for success
Boys’ lacrosse: With a different level of energy and focus, MV feels primed for success Baseball: Concord makes eight errors but shows reasons for optimism in wild extra-inning loss
Baseball: Concord makes eight errors but shows reasons for optimism in wild extra-inning loss Opinion: Members of NH Jewish community write letter to NH congressional delegation
Opinion: Members of NH Jewish community write letter to NH congressional delegation Opinion: Whatever a court decides, Elizabeth Gurley Flynn retains an important place in American labor history
Opinion: Whatever a court decides, Elizabeth Gurley Flynn retains an important place in American labor history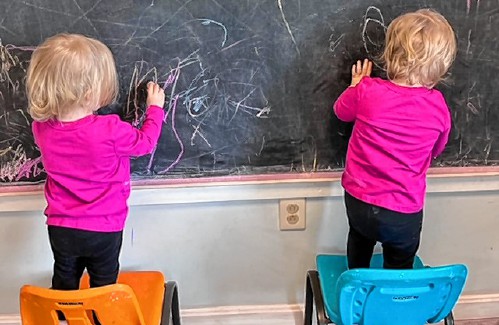 Opinion: How our twin toddlers turned our lives (and chairs) upside down
Opinion: How our twin toddlers turned our lives (and chairs) upside down Sunapee Kearsarge Intercommunity Theater presents ‘Olympus On My Mind’ in April
Sunapee Kearsarge Intercommunity Theater presents ‘Olympus On My Mind’ in April
