Latest News
 Big drop in tuition and aid is boosting Colby-Sawyer
Big drop in tuition and aid is boosting Colby-Sawyer
 College due process rights at issue in NH bill
College due process rights at issue in NH bill
 Opinion: Inherited hatred and how to stop it
Opinion: Inherited hatred and how to stop it

Concord fights stubborn brush fire along bank of Merrimack River
The Concord Fire Department spent Wednesday morning fighting a stubborn brush fire burning on a steep bank between the Merrimack River and the rail line just south of Sewalls Falls Road.“We think it had been smoldering for a couple of days,” said...

New Hampshire residents weigh compassion and caution in medical aid in dying bill
Phillip Kaneb, sporting a vibrant blue shirt with a sticker boldly stating “no suicide,” strolled around the State House’s hallways on Wednesday, pausing intermittently to admire the paintings hanging on the walls, his infectious smile bringing joy to...
Most Read
Editors Picks
 What’s in a name? Ask an Epsom Yeaton.
What’s in a name? Ask an Epsom Yeaton.
 Downtown cobbler breathes life into tired shoes, the environmentally friendly way
Downtown cobbler breathes life into tired shoes, the environmentally friendly way
 People of color incarcerated at higher rates in New Hampshire, but data is limited
People of color incarcerated at higher rates in New Hampshire, but data is limited
 Former Concord firefighter sues city, claiming years of homophobic sexual harassment, retaliation
Former Concord firefighter sues city, claiming years of homophobic sexual harassment, retaliation
Sports

High schools: Bow’s Kelly lifts Falcon softball to victory in walk-off, more Monday results, plus Saturday’s track meets
SoftballBow 5, Campbell 4Key players: Bow – Maddy Oppold (2-for-4), Ella Roos (3-for-4), Taylor Ouellette (2-for-4, 2 RBI), Emma Kelly (1-for-4, walk-off single), Lilly Wright (1-for-3, 2-run HR), Caly Poitras (4 IP, 7 H, 1 BB), Taylor Ouellette (3...
 Patriots’ draft approach likely to be different in post-Belichick era
Patriots’ draft approach likely to be different in post-Belichick era
 High schools: Weekend results
High schools: Weekend results
Opinion

Opinion: NH youth’s effort for a more robust climate curriculum
Elisabeth Bialosky, Youth Campaign Organizer/350NH. Rep. Wendy Thomas, Town of Merrimack. The kids are angry. And they have every right to be.Last summer students and youth advocates concerned about climate change had an idea: “Could we work on a...
 Opinion: This stinks, but if you don’t speak up it’s going to reek
Opinion: This stinks, but if you don’t speak up it’s going to reek
 Opinion: The problem with Ukraine
Opinion: The problem with Ukraine
 Opinion: This Earth Day, help keep local land clean and healthy
Opinion: This Earth Day, help keep local land clean and healthy

Politics

Charities will not have to pay rent to casinos under new law
Deb Leahy can finally breathe easy, freed from the fluctuating rental fees each time her organization partners with a New Hampshire casino for donations.Thanks to a new law, casinos are now prohibited from imposing rent charges on charities for...
 Sununu says he’ll support Trump even if he’s convicted
Sununu says he’ll support Trump even if he’s convicted
 NH mayors want more help from state on homelessness prevention funds
NH mayors want more help from state on homelessness prevention funds
 Two democrats with parallel views run for same State Senate seat
Two democrats with parallel views run for same State Senate seat
 House passes bill removing exceptions to state voter ID law
House passes bill removing exceptions to state voter ID law
Arts & Life

Community Players of Concord present newest adaptation of Pride and Prejudice
“Not Your Grandmother’s Jane Austen…”Bold, surprising, boisterous, and timely, this Pride and Prejudice for a new era explores the absurdities and thrills of finding your perfect (or imperfect) match in life.The outspoken Lizzy Bennet is determined to...
 Vintage Views: From darkness to light
Vintage Views: From darkness to light
 From the farm: Spring brings calves, beautiful calves
From the farm: Spring brings calves, beautiful calves
Obituaries
 Elizabeth G. Mahon
Elizabeth G. Mahon
Elizabeth "Betty" G. Mahon Boscawen, NH - Elizabeth "Betty" G. Mahon, age 93, passed away peacefully on Saturday, April 13, 2024 with family by her side. She was born in Salem, MA daughter of the late John and Elizabeth (Tansey) Gannon.... remainder of obit for Elizabeth G. Mahon
 Marylee Meagher
Marylee Meagher
Hudson, NH - Marylee Meagher, 67, of Hudson, passed away Friday, April 19, 2024 at Southern NH Medical Center surrounded by her family and friends. She was born on October 15, 1956 in Nashua, daughter of the late Edgar and Agnes (Le... remainder of obit for Marylee Meagher
 Leonard Buxton
Leonard Buxton
Henniker, NH - Leonard N. Buxton age 72 passed on from this world on April 19th 2024, after a two decade battle with cancer, at his beloved farm in Henniker NH. He manufactured the "Buxton Burners' woodstove. He was one of the first ven... remainder of obit for Leonard Buxton
 Robert A. Laferriere
Robert A. Laferriere
Boscawen, NH - Robert A "Bob" Laferriere passed away peacefully after a brief battle with cancer on Thursday, April 18, 2024, surrounded by family at his residence in Boscawen, NH. He was born on June 18, 1959, in Manchester, NH, and w... remainder of obit for Robert A. Laferriere

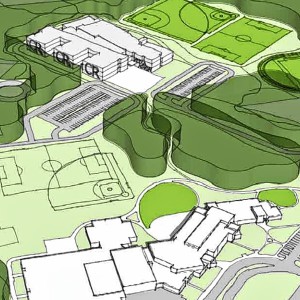 Middle School Project budget will be set in July, new timeline reveals
Middle School Project budget will be set in July, new timeline reveals
 State police: Manchester police officer arrested on multiple domestic violence-related charges
State police: Manchester police officer arrested on multiple domestic violence-related charges
 Bill requiring educators to answer parents’ questions moves forward
Bill requiring educators to answer parents’ questions moves forward
 Concord police ask for help in identifying person of interest in incidents of cars being keyed during Republican Party event
Concord police ask for help in identifying person of interest in incidents of cars being keyed during Republican Party event
 Construction of housing project in former Church to begin with parking dispute in the rearview
Construction of housing project in former Church to begin with parking dispute in the rearview
 High schools: Coe-Brown softball wins 5th straight, Concord’s McDonald pitches first varsity win, Tide’s Doherty scores 100th career point
High schools: Coe-Brown softball wins 5th straight, Concord’s McDonald pitches first varsity win, Tide’s Doherty scores 100th career point

 Gallery: Forty-mile gravel bicycle race draws racers from all over the region
Gallery: Forty-mile gravel bicycle race draws racers from all over the region Wednesday’s high schools: Fancher’s 2-run blast leads Concord baseball to victory; plus more baseball, softball, lax and tennis results
Wednesday’s high schools: Fancher’s 2-run blast leads Concord baseball to victory; plus more baseball, softball, lax and tennis results Opinion: Student power: Confronting collaborators and cowards at home and abroad
Opinion: Student power: Confronting collaborators and cowards at home and abroad Concord Monitor editor Mike Pride’s final book explores the lives, works of Northern New England poets
Concord Monitor editor Mike Pride’s final book explores the lives, works of Northern New England poets Active Aging: John Burke of Peterborough celebrated his 81st birthday with 81 hikes up Pack Monadnock
Active Aging: John Burke of Peterborough celebrated his 81st birthday with 81 hikes up Pack Monadnock
